Name: Ng Ming En
Complaints : Severe vomiting, diarrhea, abdominal cramps
Diagnosis : Food poisoning
Reason / Rationale provided why bacterial food poisoning is suspected over other types of microorganisms associated food poisoning:
• Abdominal cramps, diarrhea, and vomiting, starting from one hour to four days after eating tainted food and lasting up to four days, usually indicate bacterial food poisoning.
• Vomiting, diarrhea, abdominal cramps, headaches, fever, and chills, beginning from 12 to 48 hours after eating contaminated food — particularly seafood — usually indicate viral food poisoning.
• Vomiting, diarrhea, sweating, dizziness, tearing in the eyes, excessive salivation, mental confusion, and stomach pain, beginning about 30 minutes after eating contaminated food, are typical indications of chemical food poisoning.
• Partial loss of speech or vision, muscle weakness, difficulty swallowing, dry mouth, muscle paralysis from the head down through the body, and vomitting may indicate botulism, a severe but very rare type of bacterial food poisoning.
http://www.webmd.com>/content>/article>/54/61474
Types of possible food poisoning causing bacteria to be ruled out (Based on onset of symptoms displayed) >
1. Bacillus cerus
Bacillus cerus causes 2 forms of food poisoning: emetic type and diarrheal type. The emetic type is manifested by nausea, vomiting, abdominal cramps, and occasionally diarrhea and is self-limiting, with recovery within 24 hours. The diarrheal type is manifested by profuse diarrhea with abdominal pain and cramps; fever and vomiting are uncommon.
In this case, the patient has complaints of severe vomiting, abdominal cramps and diarrhea.
Hence, food poisoning caused by Bacillus cerus is ruled out based on the above explanation of the onset of symptoms.
2. Campylobacter jejuni
http://peer.tamu.edu>/curriculum_modules>/Environ_Hazard>/module_2>/lesson4.htm
Food poisoning caused by this bacteria may cause mild or severe diarrhea, often with fever and traces of blood in the stool.In this case, the patient does not have fever. As such, food poisoning caused by Campylobacter jejuni is ruled out.
3. Clostridium botulinum
As stated, the patient suffers from gastrointestinal complaints. However, should a person be infected by this microorganism and has botulism, gastrointestinal symptoms are not regularly prominent. Instead, symptoms like visual disturbances, inability to swallow, and speech difficulty should arise.As such, food poisoning caused by Clostridium botulinum is ruled out.
4. Listeria
In most cases, Listeria infection causes fever and influenza. Here, the patient does not suffer from fever or influenza. Hence, food poisoning caused by Listeria is ruled out.
http://peer.tamu.edu>/curriculum_modules>/Environ_Hazard>/module_2>/lesson4.htm
5. Salmonella
Salmonella causes nausea, vomiting, cramps, fever, and diarrhea within 48 hours of eating the offending food.Since the patient has no complaints of having fever, food poisoning caused by Salmonella is ruled out.
http://www.pdrhealth.com>/patient_education>/BHG01GA29.shtml
6. Shigallae sp.
The usual clinical findings of a patient who has food poisoning due to shigallae sp. should present a sudden onset of abdominal pain, fever, and watery diarrhea. In more than half of adult cases, fever and diarrhea subside spontaneously in 2 to 5 days. In this case, Ng Ming En suffers from severe vomiting, but this is not associated with food poisoning caused by shigallae sp.Also, most cases of shigella infection occur in children under 10 years old. Ng Ming En is 33 years old.Hence, food poisoning caused by Shigallae sp. is ruled out.
7. Vibrio cholera
Vibrio cholera affects the intestinal tract. It also causes mild to severe diarrhea, vomiting, and dehydration.Since the patient does not complain of dehydration, food poisoning caused by Vibrio cholera is ruled out.
http://peer.tamu.edu>/curriculum_modules>/Environ_Hazard>/module_2>/lesson4.htm
With the elimination of above possible casaultive agents, the most likely cause of food poisoning in this patient is :
1. Staphylococcus aureus
Staphylococcal food poisoning is the most common form of food poisoning.
“some foodborne diseases are caused by the presence of a toxin in the food that was produced by a microbe in the food. For example, the bacterium Staphylococcus aureus can grow in some foods and produce a toxin that causes intense vomiting.”
Besides causing severe vomiting, Staphylococcus aureus also causes diarrhea and abdominal cramps. These are the exact complaints of the patient.
http://www.cureresearch.com>/f/food_poisoning>/subtypes.htm
Laboratory Diagnosis (S.Aureus)
Microscopy
On gram staining, gram positive and cocci-shaped colonies are found, which are arranged in irregular clusters.

Adapted from:
http://www.life.umd.edu>/classroom>/bsci424>/PathogenDescriptions>/StaphylococcusImages.htm
Culture
The purpose of this test is to isolate bacteria or other organisms that might be causing the symptoms so they can be identified.
Blood agar supports the growth of most bacteria including Staphylococcus aureus, Listeria monocytogenes, and yeast, which are infrequently implicated in food poisoning or gastrointestinal infections, but do not grow on the other media.
Upon culturing on blood sheep agar, Staphylococcus aureus, if present, shows beta haemolytic characteristics, and yield yellow or gold colonies.

Adapted from : http://eyemicrobiology.upmc.com>/Bacteria.htm
Typical test characteristics of S. aureus:
Catalase activity- Positive
Coagulase production- Positive
Thermonuclease production- Positive
Lysostaphin sensitivity- Positive
Anaerobic utilization of mannitol- Positive
Anaerobic utilization of glucose- Positive
http://www.cfsan.fda.gov>/~ebam>/bam-12.html
Biochemical Tests
http://dentistry.ouhsc.edu>/intranet-web>/courses>/dmi_8351>/Catalase.html
1. Catalase Test
Principle
Hydrogen peroxide (H2O2) is decomposed by catalase enzyme to water and oxygen. Bacteria are unable to protect themselves from the lethal effect of hydrogen peroxide, which is accumulated as a product of carbohydrate metabolism. Catalase is a hemoprotein. Catalytic decomposition of hydrogen peroxide leads to the reduction of ferric iron (Fe3+) to ferrous iron (Fe2+) and the re-oxidation of the latter by oxygen. This reaction can be summarized by the following equation:
Catalase
H2O2 --------------------------> 2H2O + O2
Procedure
• A clean microscope slide is labeled for the organism to be tested.
• One drop of 3% H2O2 is added to the slide.
• Several isolated colonies of the organism is transferred to the H2O2 on the microscope slide using a sterile inoculating loop.
Caution
Avoid touching the surface of the media plates containing sheep or human blood, as red blood cells have catalase activity and will give a false-positive result.
Interpretation of positive test: Evolution of gas bubbles
Negative test: No gas bubbles

2. Coagulase Test
Principle
The coagulase test serves to differentiate Staphylococcus aureus from Staphylococcus epidermidis. Should coagulase be present, a clot will be formed in a tube of citrated platelet-rich plasma (~ >150 x 106 platelets/cc plasma). The citrate, which is an anti-coagulant, is added to avoid auto-clotting.
Procedure
• A generous loopful of stool is added to a tube of citrated rabbit plasma.
• Using the loop, the inoculum is thoroughly homogenized
• The tube is then incubated at 37o C for 1 to 4 hours.
• The tube is observed at 30 minute to hourly intervals for the first 2 hours to detect the presence of a clot by tipping the tube gently on its side.
• Should clotting be seen within 24 hours, the coagulase test is considered positive.
http://www.life.umd.edu>/classroom>/bsci424>/LabMaterialsMethods>/CoagulaseTest.htm
Antibiotic Susceptibility Test
1. Agar Disk Diffusion Method• With a sterile loop, the tops of four to five colonies of S. aureus from pure culture are picked up.
• The colonies were suspended in 5 ml of sterile physiologic saline.
• The inoculum turbidity is standardized to equivalent of a 0.5 McFarland standard.
• The entire surface of a Mueller-Hinton agar plate is inoculated using a sterile swab.
• Disks containing 10 µg of penicillin, 10 µg of ampicillin, 30 µg of cephalexine, 1 µg of oxacillin, and 30 µg of kanamycin are then placed using a sterile forcep onto the agar surface and gently pressed down to ensure contact.
• Plates are incubated at 35°C for 20 h.
• Subsequently, the diameter of the zone of inhibition around each disk is measured.
http://jds.fass.org>/cgi>/content>/full>/86/10/3157
Treatment Therapy
The larger the zone diameter, the more sensitive the bacteria is to the antibiotic. Through this test, it is observed that the largest zone diameter is seen around Penicillin G disk. Hence, Ng Ming En can be treated using Penicillin G antibiotic.
Case study 2
Patient Name: Kwan Siew Lan
Complaints: Diarrhea
Diagnosis: Enterocolitis
Reasons why the microorganisms were excluded:
•Staphylococcus aureus and Bacillus cereus
These organisms produce enterotoxins in food, causing nausea and vomitting – and to a much lesser extent diarrhea. Since these 2 organisms are more likely to cause nausea and vomitting instead of diarrhea, they can be ruled out since the patient’s symptom is only diarrhea.
• Clostridium difficile
Clostridium difficile is a common infection that is acquired while in the hospital that causes diarrhea. However, it is ruled out because it usually causes Pseudomembranous colitis and not enterocolitis.
http://en.wikipedia.org>/wiki>/Clostridium_difficile
Escherichia coli
E. coli is a common cause of diarrhea. Of particular interest is the E. coli O157:H7, a strain of E. coli that produces a toxin that causes hemorrhagic enterocolitis (enterocolitis with bleeding). However, patient is diagnosed with only enterocolitis, but not hemorrhagic enterocolitis, and therefore this organism is ruled out.
http://www.medicinenet.com>/diarrhea>/page3.htm
• Shigella Flexneri
Shigella sp. are the principal agents of dysentery. This disease differs from profuse watery diarrhea, as the dysenteric stool is scant and contains blood, mucus, and inflammatory cells. The patient is only reported of having diarrhea and not dysenetery as a symptom, therefore this microorganism was ruled out.
http://gsbs.utmb.edu>/microbook>/ch022.htm
Other microorganisms
The other organisms such as vibrio cholerae, entamoeba hitolytica, rotavirus, and Norwalk virus and are found to only cause diarrhea and other gastrointestinal symptoms such as nausea, vomiting and abdominal cramps. These organisms do not have any relations in causing enterocolitis.
http://en.wikipedia.org>/wiki>/Norwalk_virus#Diagnosis_of_human_illness
http://vm.cfsan.fda.gov>/~mow>/chap23.html
http://en.wikipedia.org>/wiki>/Rotavirus
Reasons why the organisms were included:
• Salmonella sp.
Salmonella sp. are known to cause enterocolitis with symptoms nausea, vomiting, diarrhea. Since this organism matches the patient’s complaints and diagnosis, the infection is very likely to be due to Salmonella sp.
• Campylobacter jejuni
This organism is known to cause enterocolitis with fever, abdominal cramps, watery to bloody diarrhea. The above symptoms are caused by toxin (endotoxin & exotoxin) produced by Campylobacter sp. Since this organism matches the patient’s complaints and diagnosis, the infection is very likely to be due to Campylobacter jejuni.
Laboratory diagnosis for Salmonella sp.
• They are facultative anaerobes
• They are Gram-negative rods
• They are non lactose fermentors
• They produce H2S
Culture
Differential medium cultures – The stool is layed onto an EMB or MacConkey agar as it permits the rapid detection of lactose nonfermenters (organisms that would grow on the plate would be salmonellae, shigellae, proteus etc). Gram positive organisms are also inhibited when these plates are used.
Selective medium cultures – The specimen would also be plated onto an XLD plate, which favours the growth of salmonellae and shigellae over other Enterobacteriaceae.
Enrichment cultures – The specimen is also placed in selenite F or tetrathionate broth which inhibit replication of normal intestinal flora and permit multiplication of salmonellae. After incubation of 1-2 days, the sample from the broth is plated onto a differential and selective media.

Salmonella sp. on XLD plate
http://www.textbookofbacteriology.net>/salmonella.html
Microscopy

Gram negative bacilli of Salmonella sp
http://dentistry.ouhsc.edu>/intranet-Web>/Courses>/DMI_8351>/Images>/170.JPEG
Biochemical reactions
• Indole test – negative
• Motility-positive
• Glucose(TSI) – positive
• Lysine decarboxylase(LIA) – positive
• H2S(TSI and LIA) – positive(blackening)
Note: Enterocolitis is self limiting in 2-3 days. Thus antibiotic treatment not required. (no antibiotic susceptibility tests)
Laboratory diagnosis for campylobacter jejuni
o Gram negative, “S” or “gull wing” shaped
o Motile with a single polar flagellum
o micro-aerophilic (5% O2 with 10% CO2)
Culture
The media that can be used are:
• Campylobacter selective media at 42º C, 10% carbon dioxide, 3-4 days incubation. The incubation atmosphere is made by placing the plates in an anaerobe incubation jar without the catalyst and to produce the gas with a commercially available gas generating gas pack.
• Skirrow medium or Campy BAP medium
Microscopy

http://education.med.nyu.edu>/courses>/old>/microbiology>/courseware>/infect-disease>/CAMPJ.gi
Biochemical tests
Catalase test - positive
Oxidase test - positive
Hippurate test - positive
Growth at 42°C - positive
Antibiotic susceptibility
Campylobacter jejuni is susceptible to erythromycin and ciprofloxacin. An inhibitory zone of more than 6 mm is considered to be susceptible.
Case study 3
Patient: Maisy Wong, Female, 66 years
Complaints: Fever, chills, bladder distension; on indwelling catheter
Diagnosis: Urinary Tract Infection
Interpretation of symptoms:
Indwelling catheter: A tube that drains urine from the bladder. The tube is placed into the urethra and up into the bladder. An indwelling catheter is used when you can't urinate normally. Indwelling catheters can cause urinary tract infections.
Bladder distension: Inability to urinate.
Old age: increased risk of women after menopause (over 65) for urinary tract infection. With estrogen loss, there is a reduction of certain immune factors in the vagina, which results in E. coli to adhere to vaginal cells. The walls of the urinary tract thin out, weakening the mucous membrane and reducing its ability to resist bacteria. The bladder may lose elasticity and fail to empty completely.
Possible suspected Microoganisms (Narrowed down)
Classification: Enterobacteriaceae – Gram negative rods, grow well on Mac-Conkey agar, grow aerobically and anaerobically (facultative anaerobes), ferment glucose with gas production, catalase-positive and oxidase-negative.
• E. coli. The gram-negative bacterium Escherichia coli is responsible for between 80% and 85% of UTI cases. In most cases of UTI, E. coli, which originates as a harmless microorganism in the intestines, spreads to the vaginal passage where it invades and colonizes the urinary tract.
• Klebsiella pneumoniae is present in the feces and occasionally causes UTI. Klebsiella species are common in hospitals ands nursing homes where they cause urinary tract infections in catheterized patients.
• Proteus mirabilis can cause urinary tract infections and hospital-acquired infections. Once attached to urinary tract, P.mirabilis infects the kidney more commonly than E. coli. During infection due to the action of urease, stones are formed composing of magnesium ammonium phosphate caliculi and calcium phosphate. As one of the most common symptoms of proteus infecting UTI is the presence of kidney stones, this microbe is being ruled out since patient do not show signs of developing kidney stones.
Suspected Diagnosis: Acute Pyelonephritis (Kidney Infection)
It refers to the inflammation of the kidney and upper urinary tract
• Common in adult females.
• Results from urine that becomes stagnant due to obstruction of free urinary flow.
• Catheters may also trigger a kidney infection.
• Symptoms include fever and chills, burning or frequent urination.
Diagnostic Confirmatory Tests ( E.Coli, Proteus mirabilis & Klebsiella)
1.Gram Stain
Gram stain is a useful procedure in differentiating the gram negative and gram positive bacteria. When examined microscopically, identification of morphology (shape, size and arrangement) can be noted. Since E.Coli, Klebsiella pneumoniae & Proteus mirabilis are all gram negative rods microbes, their morphological appearance should be pink in color after gram staining.

Adapted From :
http://biology.clc.uc.edu>/fankhauser>/Labs>/Microbiology>/Gram_Stain>/Gram_stain_images>/index_gram_stain_images.html
2. Culture on selective media – Mac-Conkey agar / Eosin-Methyl-Blue (EMB agar) / Blood agar
A urine culture is a urine specimen observed 24 to 48 hours in a laboratory for the presence of any bacterial growth. Preventing growth of other microorganisms, Mac-Conkey agar or EMB are selective agar media for enteric gram-negative rods – E.coli, Klebsiella. An isolate from urine can be identified as E.coli by its hemolysis on blood agar.

MacConkey Agar - Escherichia coli (lactose fermenting) (at left) and non-lactose-fermenting Proteus (at right). Lactose-fermenting bacteria appear bright pink, while non-lactose-fermenting bacteria appear colorless

E. coli - on EMB, showing a metallic green sheen

E. coli - on blood agar - non hemolytic
Adapted from :
http://faculty.mc3.edu>/jearl>/ML>/ml-12-2.htm
3.Biochemical Identification Tests
1. Oxidase Test
2. Indole Production test
3. Methyl Red
4. Voges-Proskauer
5. Citrate Test
6. Triple Sugar Iron test
1. Oxidase Test (Principle)
This oxidase test differentiates those that possess the enzyme cytochrome oxidase C from those that lack the enzyme
Lab procedures
1. Dip a filter paper strip in oxidase reagent to soak about ¼ of its length.
2. Transfer some cells from a bacterial colony using a toothstick and smear them on the reagent-soaked portion of the filter paper strip.
Intepretations
A positive oxidase test is indicated by development of dark purple color at the site of the smear within seconds. An oxidase negative test shows no color change. Gram negative rod (E.Coli, Klebsiella pneumoniae) should show no color change
2. Indole Test (principle)
The indole test is used to measure the ability of the bacteria to hydrolyze and deaminate trytophan with the production of indole, pyruvic acid & ammonia..
Lab procedures
1. Inoculate the organism into peptone water broth, incubated at 37ºC for 24 hours.
2. Add 5 drops of kovac’s reagent down the inner wall of each of the tube culture
Intepretations
The presence of indole production is indicated by development of a red color at the interface of the reagent and the broth within second after adding the reagent. E.coli should be positive for indole test while Klebsiella pneumoniae should appear negative
3. Methyl Red test (Principles)
Many species of the enterobacteriaceae family produce strong acids from glucose via mixed acid fermentation pathway. Only species that produce sufficient acid as a result of carbohydrate fermentation can maintain pH at below 4.4 against the buffer system of the test medium after prolonged incubation. These species are methyl red positive.
Lab procedures
1. Inoculate organism into MR-VP broth, incubate at 37ºC for 48hours.
2. Add 5 drops of methyl red reagent directly into each of the broth culture.
Intepretations
A positive methyl red test is shown be the development of a stable red color in the surface layer of the medium. Organisms that produce lesser quantities of acid from the test substrate give an intermediate orange color between the yellow and red; such color change is read as negative test. E.Coli should appear positive while Klebsiella pneumoniae should appear negative.
4. Voges-Proskauer test (Principles)
Many bacteria ferment glucose to form pyruvic acid. Pyruvic acid is further metabolized via a number of metabolic pathways dependent upon the enzyme system possessed by different bacteria. The Voges-Proskauer reaction test is based on the conversion of acetoin to a red colored complex through the action of KOH, atmospheric O2 and α-napthol.
Laboratory procedures
1. Inoculate organisms into MR-VP broth, incubate at 37ºC for 24hours.
2. Transfer 1ml of each broth culture into a clean test tube.
3. Add 0.6ml of 5% α-napthol followed by 0.2ml of 40% KOH.
4. Shake the tubes gently to expose the medium to atmospheric oxygen. Leave it undisturbed for 10-15mins.
Intepretations
In a positive Voges-Proskauer reaction test, a red color develops at the surface of the medium after 15minutes following the addition of the reagents A negative reaction test shows no color change in the medium. E.Coli should appear negative for this while Klebsiella pneumoniae should appear positive for this.
5.Citrate Utilization test (Principles)
Apart from utilization of carbohydrate for energy, some bacteria can obtain energy by utilizing citrate as sole carbon source. The medium used to detect citrate utilization must be devoid of protein and carbohydrates as source of carbon. The citrate utilization by the bacteria turns the medium alkaline due to the production of ammonia. A positive citrate utilization test is indicated by the bromothymol blue indicator turning from green to blue.
Lab procedures
1. Streak with a stab needle a colony of each microorganisms on the Simmons Citrate slant and stab the remaining of the culture into the agar slant.
2. Incubate the inoculated slants at 37ºC for 24hours.
Intepretations
A positive citrate utilization test shows a color change from green to blue. E.Coli should appear negative for this test while Klebsiella pneumoniae should appear positive for this test.

Voges Proskauer test

Methyl red

Indole test

Citrate test
http://www.mc.maricopa.edu>/~johnson>/labtools>/Dbiochem>/imvic.html
6. TSI test (principles)
Triple sugar iron agar is used primarily for the identification of bacterial strains / species of the Enterobacteriaceae family. This differential medium is used to determine carbohydrate utilization (glucose, lactose & sucrose) and hydrogen sulfide (H2S) gas production. Fermentation of glucose, lactose and / or sucrose produces relatively large quantities of acid because of the higher concentration of sucrose and lactose in the medium.This quantity of acid is sufficient to overcome the alkaline reaction evolving in the slant and entire tube remains yellow in color. Under acidic conditions, H2S- producing bacteria produce H2S which reacts with ferrous sulphate in the medium to form an insoluble black ppt, ferrous sulphide (FeS).
Lab procedures
1. Stab-inoculate each test organism into a TSI agar tube.
2. After stabbing, streak the remaining organisms on the agar slant surface with a back and forth motion
3. Incubate the tubes at 37ºC for 24hours.
Intepretations
Fermentation of glucose only ------ (alkaline slant / acid deep) (Red slant / yellow butt)
Fermentation of glucose, lactor and / or sucrose ------ (acid slant / acid deep) (yellow slant / yellow butt)
No carbohydrate fermentation ------- (Alkaline slant / Alkaline deep) (Red slant / Red butt)
Black precipitate (ppt) ----- H2S production.
Both Klebsiella pneumoniae & E.Coli should show acid slant/acid butt with no H2S production.
*Overall summary for Biochemical test*
For Klebsiella pneumoniae --++ (IMViC tests)
For E.Coli ++-- (IMViC tests)
Disk Diffusion Susceptibility Test• Penicillin
• Ampicillin
• Gentamicin
• Erythromycin
• Ceftazidime
References
http://www.med.umich.edu>1libr>aha>aha_urincath_crs
http://www.reutershealth.com>wellconnected>doc36
http://www.findarticles.com>particles>mi_g2601>is_0011>ai_2601001150
Case study 4
Name : Wong Fei Hong , 67yrs old, Male
Complaints : Fever, chills, bladder distension ; on indwelling catheter
Diagnosis : Urinary Tract Infection
Suspected diagnosis:
The incidence of UTI in men begins to increase with age, particularly after age 50 years. Also, the spectrum of causative agents is somewhat broader in these elderly men.10
This patient is suffering from complicated lower Urinary tract infection (UTI). 'Lower UTI' implies infection of the bladder (and possibly the urethra). 11 Complicated UTI indicates a urinary tract infection that occurs in a patient with a structural or functional abnormality of the genitourinary tract such as indwelling catheter.1 This makes the patient very vulnerable to infections.
Possible suspected microbes (Narrowed down)
In complicated cases of UTI, the most common causes of UTI are E. coli, Klebsiella pneumoniae, Proteus mirabilis, Enterococcus sp., Pseudomonas aeruginosa.(2)
Proteus mirabilis is a Gram-negative, facultatively anaerobic bacterium.(3) It is mostly reported in individuals with structural abnormalities of the urinary tract and is frequently isolated from the urine of elderly patients undergoing long-term catheterization and women with repeated UTI.(4)
Klebsiella pneumoniae is a Gram-negative bacterium, a cylindrical rod of size about 2 microns by 0.5 microns, thus much smaller than the cells of higher organisms such as humans.5 It is more commonly implicated in hospital-acquired urinary tract.6 Klebsiella pneumoniae ranks second to E. coli for urinary tract infections in older persons.(6)
Pseudomonas aeruginosa is a Gram-negative, aerobic, rod-shaped bacterium with unipolar motility.(7) Urinary tract infections (UTI) caused by Pseudomonas aeruginosa are usually hospital-acquired and related to urinary tract catheterization, instrumentation or surgery.(8) Pseudomonas aeruginosa is the third leading cause of hospital-acquired UTIs.(8)

Gram stain of Pseudomonas aeruginosa cells
Adapted from:
http://textbookofbacteriology.net>/pseudomonas.html
E. coli is a non-spore-forming, Gram-negative, rod-shaped bacteria.(9) E. coli is still the most common organism in complicated UTIs.(1) It is found in roughly equal proportions in elderly men and women.(9)
Enterococci are Gram-positive cocci which often occur in pairs (diplococci).12Enterococcus is a frequent pathogen in older patients.(10) Enterococci occasionally are transmitted by medical devices.(13)

http://en.wikipedia.org>/wiki>/Enterococcus
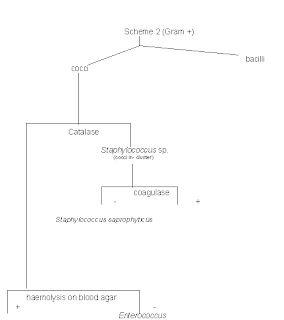
Staphylococcus saprophyticus is another common microorganism that causes UTI. However, it is more commonly seen in women of sexually active age. Thus for this case, Staphylococcus saprophyticus is highly impossible for the patient is a old male.
Laboratory diagnostic tests
1. Gram staining
Gram stain is a useful procedure in differentiating the gram negative and gram positive bacteria. When examined microscopically, identification of morphology (shape, size and arrangement) can be noted. Since E. coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa are all gram negative rods microbes, their morphological appearance should be pink in color after gram staining if the actual microorganism residing in the patient is any of these 4 gram negative rods. However, if the microbe is Enterococcus sp, it should appear blue-black to purple after gram staining
2. Culture on selective media – Mac-Conkey agar / Eosin-Methyl-Blue (EMB agar) / Blood agar
Types of agars
Blood Agar:
1. E. coli – Large gray colonies, no clearing seen
2. Klebsiella pneumoniae
3. Proteus mirabilis – no growth seen, distinct rings of swarming growth can be seen in low moisture medium
4. Enterococcus – white colonies
5. Pseudomonas aeruginosa – brown colonies, semi clearing
MacConkey Agar:
1. E. coli – Pink colonies, clearing seen (able to ferment lactose)
2. Klebsiella pneumoniae – Pink colonies (inability to ferment lactose)
3. Proteus mirabilis – colourless (inability to ferment lactose)
4. Enterococcus – colourless (inability to ferment lactose)
5. Pseudomonas aeruginosa – colourless (inability to ferment lactose)
Nutrient Agar : E. coli – white colonies
Pseudomonas aeruginosa – blue-green colonies
3.Biochemical Identification Tests
1. Oxidase Test
2. Indole Production test
3. Methyl Red
4. Voges-Proskauer
5. Citrate Test
6. Triple Sugar Iron test
*The details of the test and interpretation can be inferred to case study 3 (Maisy) since the same group of gram negative rods ( Klebsiella pneumoniae & E.Coli ) are also included as most possible suspected microbes for this case study. The only differences for this case study from maisy (Case study 3) is that there are 2 more suspected types of microbes namely Pseudomonas aeruginosa & Proteus mirabilis.
Pseudomonas aeruginosa should be oxidative positive while Proteus mirabilis should be oxidative negative. Overall summary of expected results for Proteus mirabilis in IMViC test is as follows : -+-+ respectively. Proteus mirabilis is also urease (+) which appears pink color on urea agar slant.

References
1.http://www.umm.edu>/patiented>/articles>/what_infectious_agents_that_cause_urinary_tract_infections__000036_2.htm
2.http://www.bact.wisc.edu>/Microtextbook>/index.php?name=Sections&req=viewarticle&artid=254&page=1
3. http://en.wikipedia.org>/wiki>/Proteus_mirabilis
4.http://www.clevelandclinicmeded.com>/DISEASEMANAGEMENT>/infectiousdisease/uti/uti.htm
5. http://www.genome.wustl.edu>/genome.cgi?GENOME=Klebsiella+pneumoniae
6. http://en.wikipedia.org>/wiki>/Klebsiella_pneumoniae
7. http://en.wikipedia.org/wiki>/Pseudomonas_aeruginosa
8. http://textbookofbacteriology.net>/pseudomonas.html
9. http://en.wikipedia.org/wiki>/Escherichia_coli
10. http://www.emedicine.com>/MED/topic2346.htm
11.http://www.prodigy.nhs.uk>/urinary_tract_infection_lower_men/extended_information/background_information
12. http://en.wikipedia.org>/wiki>/Enterococcus
Case study 5
Name : Khong Fay Fay, 26 yrs old Female
Complaints : Fever, chills, dysuria
Diagnosis : Urinary Tract infection
Suspected Diagnosis : acute pyelonephritis
In more than 80 percent of cases of acute pyelonephritis, the etiologic agent is Escherichia coli (gram negative bacilli). Other etiologic causes include aerobic gram-negative bacteria, Staphylococcus saprophyticus (gram positive cocci), and enterococci (gram positive cocci). The microbial spectrum associated with different types of urinary tract infections (UTIs) is wide
(from: http://www.aafp.org>/afp>/20050301/933.html )
The most common cause of pyelonephritis is the backward flow of bacterial infected urine from the bladder to the upper urinary tract. The bacteria that are most likely to cause pyelonephritis are those that normally occur in the feces. E. coli causes about 85% of acute bladder and kidney infections in patients with no obstruction or history of surgical procedures. Klebsiella (gram negative bacilli), Enterobacter (gram negative bacilli), Proteus (gram negative bacilli), or Pseudomonas (gram negative bacilli) are other common causes of infection. Once these organisms enter the urinary tract, they cling to the tissues that line the tract and multiply in them.
From:http://www.austincc.edu>/lesalbin>/Chapter%2026.htm and http://www.kcom.edu>/faculty>/chamberlain>/Website>/lectures>/lecture>/uti.htm
Possible suspected microorganisms
For our case, the most likely suspected microorganisms that cause UTI is E.coli and S. saprophyticus. The rationale that we are choosing this two microorganism is because E.coli is the most common microorganism that causes UTI in women while S. saprophyticus is the second leading microorganism that causes pyelonephritis in young sexually active women.
The reasons why the other microorganism is being eliminated is because they are less common. The other reasons are:
Klebsiella ranks second to E. coli for urinary tract infections in older persons. Not in young women
An alkaline urine sample is a possible sign of Proteus mirabilis. This bacterium has the ability to produce high levels of urease. Urease hydrolyzes urea to ammonia and thus makes the urine more alkaline. If left untreated, the increased alkalinity can lead to the formation of crystals of struvite, calcium carbonate, and/or apatite or kidney stones.
Laboratory diagnostic test• For E.Coli, the same confirmatory testing are performed as reflected in case 3 biochemical test (IMVIC), gram staining, oxidase test
*Intepretations for each test are reflected in case 3 study.
• For S. saprophyticus, laboratory diagnostic test can include oxidase test, culture on selective media such as MacConkey or EMB, biochemical testing (IMVIC)
Lab diagnosis
S. saprophyticus appear to be non lactose fermentor when culture on MacConkey agar or EMB. Their colonies should appear colorless. (Image can be found at case study 3, under Culture on selective media). Their biochemical test profile should be oxidase positive, citrate positive, indole negative, TSI negative.


Treatment
The standard treatment for uncomplicated pyelonephritis is a 14-day course of oral antibiotics, usually trimethoprim-sulfamethoxazole (TMP-SMX) or a fluoroquinolone. Sometimes patients with uncomplicated pyelonephritis are first given an antibiotic injection, if indicated.
Oral amoxicillin or amoxicillin-clavulanate (Augmentin) may be prescribed for women with bacteria that do not respond to standard regimens (e.g., gram-positive organisms, including Enterococcus species and S. saprophyticus).
From: http://adam.about.com>/reports>/000036_7.htm